C-Nucleosides: Difference between revisions
(Created page with "'''''C''-Nucleosides''' In ''C''-nucleosides, a carbon-carbon bond links the nucleobase (or nucleobase analog) to the sugar. This is in contrast to canonical nucleosides, where a nitrogen atom links the base to the ribose or 2'-deoxyribose. The best-known natural ''C''-nucleoside is pseudouridine. Several therapeutic nucleosides (or their prodrug forms used as active pharmaceutical ingredients) are known that are ''C''-nucleosides. Review M. Hocek, ''C''-Nucleoside...") |
(Added a depiction of the general structure of a C-nucleoside.) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== ''C''-Nucleosides == | |||
[[File:General structure c-nucleoside.png|thumb|right|General structure of a C-nucleoside featuring D-ribose as the sugar unit. Note the C-C bond that links the nucleobase to the sugar.]] | |||
In ''C''-nucleosides, a carbon-carbon bond links the nucleobase (or nucleobase analog) to the sugar. This is in contrast to canonical nucleosides, where a nitrogen atom links the base to the ribose or 2'-deoxyribose. The best-known natural ''C''-nucleoside is pseudouridine. Several therapeutic nucleosides (or their prodrug forms used as active pharmaceutical ingredients) are known that are ''C''-nucleosides. | |||
== References == | |||
=== Review === | |||
[1] M. Hocek, ''C''-Nucleosides: synthetic strategies and biological applications. ''Chem. Rev''. '''2009''', ''109'', 6729–6764. https://doi.org/10.1021/cr9002165 | |||
=== Synthetic Papers === | |||
[2] H.-J. Kim, N. A. Leal, S. Hoshika, S. A. Benner, Ribonucleosides for an artificially expanded genetic information system. ''J. Org. Chem''. '''2014''', ''79'', 3194−3199. https://doi.org/10.1021/jo402665d | |||
[3] T. Gniech, C. Richert, Diastereoselective synthesis of pyridone ''ribo''-''C''-nucleosides via Heck reaction and oxidation. ''Eur. J. Org. Chem.'' '''2024''', e202400342. https://doi.org/10.1002/ejoc.202400342 | |||
Latest revision as of 15:00, 3 September 2024
C-Nucleosides
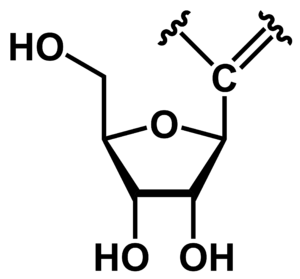
In C-nucleosides, a carbon-carbon bond links the nucleobase (or nucleobase analog) to the sugar. This is in contrast to canonical nucleosides, where a nitrogen atom links the base to the ribose or 2'-deoxyribose. The best-known natural C-nucleoside is pseudouridine. Several therapeutic nucleosides (or their prodrug forms used as active pharmaceutical ingredients) are known that are C-nucleosides.
References
Review
[1] M. Hocek, C-Nucleosides: synthetic strategies and biological applications. Chem. Rev. 2009, 109, 6729–6764. https://doi.org/10.1021/cr9002165
Synthetic Papers
[2] H.-J. Kim, N. A. Leal, S. Hoshika, S. A. Benner, Ribonucleosides for an artificially expanded genetic information system. J. Org. Chem. 2014, 79, 3194−3199. https://doi.org/10.1021/jo402665d
[3] T. Gniech, C. Richert, Diastereoselective synthesis of pyridone ribo-C-nucleosides via Heck reaction and oxidation. Eur. J. Org. Chem. 2024, e202400342. https://doi.org/10.1002/ejoc.202400342